How to Make and Grow Your Own Crystal Geodes - Cool Science Experiment for Kids - These super easy and simple instructions are based on Martha Stewart's but much better with more science background. Use natural or plastic eggshells to make beautiful, fun crystals for Easter, science fair, or any time!

Growing crystals is a simple process, ripe with science concepts, but it is not a quick process. I questioned whether Grace was ready for a process requiring so much patience.
Then I saw this pinned on Pinterest:

Come on now. If you knew how to make those, would you be able to resist them? The same day I saw the picture, I ordered the necessary supplies and got to work with my girls.
These are from Martha Stewart. Mine aren't as pretty as Martha's, but I think they're cooler because our crystals are much nicer.
Growing Crystal Geodes - A Cool Science Experiment for Kids
I'm not sure about calling this an experiment. It's more like a recipe. Anyway, read on.

Materials
- Alum powder
- You have to make sure you get the right kind of alum powder, potassium aluminum sulfate. Some commercial alum contains potassium, and some doesn't. If you get the kind without potassium, your crystals will not grow. I got mine from Talas, the company recommended on the original website. Theirs will for sure work.
- You also don't need 5 pounds. I've made a dozen crystal eggs, and I've only used 2 pounds or so.
- Eggshells or plastic eggs
- We used a combination of real eggshells, broken in half, and plastic Easter eggs. You can scrutinize our finished eggs in the slideshow below, but I don't think there was a difference between the crystals grown in the plastic and real eggs. They all came out really well.
- The geodes would look better if you cut the egg lengthwise, but have you ever tried to break an egg lengthwise? It's pretty close to impossible. Joe did blow out an egg and cut it in half lengthwise with his Dremel thing, but that's a lot of work. I guess if you want to make perfect geodes or if you're doing this for a science project, you might want to go to those great lengths, but we were just doing this for fun (and to share with you).
- Glue
- I'm pretty sure that any old glue will work. We used cow glue (that's what Grace calls Elmer's).
- You're going to coat the inside of every eggshell or plastic egg with glue, so you'll need a lot of it.
- Paintbrush
- You only need this to paint on the glue.
- Don't forget to wash the glue off before it dries. If you forget, you might as well throw the paintbrush away. Ask me how I know.
- A box or something else to put your eggs in to dry
- Wet alum crystals are very delicate. In fact, you may knock some crystals off when you pick the wet geode up out of the solution. You will want some kind of container that will allow each geode to dry without being jostled.
- The box we used was sent to us by International Delight, the creamer company. I don't know if you can buy these boxes in the store or not. It was really handy both for drying and for storing our geodes.
- If you're having trouble coming up with something, you could cut rings off of a toilet paper roll, and set the wet eggs in the rings. The rings would be about the right size and shape, and they would hold the geodes up off of the table or countertop.
- A 4-cup bowl (not pictured) - This is to mix the solution in.
- A spoon or whisk (not pictured) - For mixing the solution
- A measuring cup (not pictured) - You will need to measure ¾ of a cup of alum powder.
Sorry that I only put the consumables in the picture. I didn't think about the bowls and stuff, but they are obviously necessary.
Before You Can Grow Crystals
This is like Step 0.
The day before you want to grow crystals, you have to prepare your eggs. You have to do it the day before to allow the glue to dry. I suppose you could just wait a few hours, but the glue really needs to be dry and hardened before you put it in the alum solution.
First, paint the eggshells with a thin layer of glue. If you want crystals to grow around the edge (for a less authentic, but cooler-looking geode), make sure to paint a little glue on the outside of the rim of the eggshell.

A thinner coat is better, but as you can see, it's not a make or break issue.

Both of my kids were thrilled to paint eggshells with glue, so I let them go at it. I did have to remove ⅔ of the glue afterward to avoid deep puddles in the bottom of each egg. Puddles won't do.
While the glue is still wet, sprinkle the egg with alum powder.

Alternately, you can fill the eggshell up with alum powder, roll it around, and dump the powder back out. I don't recommend this method, but it's what we did for most of our eggs. My kids are too little to understand "sprinkle;" they're dumpers.
Don't neglect the glue on the outside of the egg. You can turn the egg over into a small bowl of alum powder to get this part.

Do this for a whole bunch of eggshells at once. It's a little messy, and you don't want to go back and do it every day or two, one eggshell at a time. That would be a serious pain in the neck.
Now We Can Grow Crystal Geodes
Okay, so you've let those eggs dry overnight. Now you're ready to make your alum solution.
If you want to use dye, stir it into 2 cups of water. We used Easter egg dye once and food coloring a couple of times. Neither of them worked particularly well. Martha Stewart recommends an expensive powdered egg dye that is supposed to get better results, but I wasn't willing to shell out the dough for it.
I liked the clear crystals best anyway.

Heat the water to almost boiling. I put mine in the microwave for 5 minutes. It think it boils during that time, but as soon as I open the microwave door, it quits. Works for me.
Pour ¾ cup of alum powder into the water and stir it for longer than you think is necessary. If there are any crystals in the bottom, you need to either stir longer or reheat the solution or both. I usually put mine back in the microwave for 2 minutes, and all the extra crystals dissolved.

The idea here is that you want a saturated solution. In other words, you want the water to be holding as much alum powder as it can possibly hold.
There's a fine line between saturated and crystals sitting around in the bottom of the bowl. If there are crystals in the bottom of the bowl, they will draw the alum away from your geodes, and the crystals in your geode won't get as big.
If there's anything else in the bowl - dust, bits of glue, unidentified floaters - strain the solution with a strainer. It's important that there aren't extraneous bits floating around in there. (I had to strain ours a couple of times.)
On the other hand, if your solution isn't saturated, when you put your alum powder-coated egg in the water, the alum powder will dissolve and your egg will be bare. That happened to one or two of our eggs. They still got crystals inside, but they didn't cover the whole egg, just the bottom where they settled out.
The good thing about this project is that it's pretty forgiving. As the solution cools, crystals are going to form on the bottom of the bowl. As long as your egg is in the bottom of the bowl, it's going to collect some crystals, even if you've messed up practically everything.
When you're satisfied that the mixture is sufficiently saturated, drop an eggshell or two into the mixture. We did all of ours two at a time.
Now, you wait.
Leave it alone until tomorrow. Pick up the eggshells tomorrow, and see how you like them. If you want the crystals to grow bigger, carefully (!!) put the eggshells back in the solution, and wait until the next day. If you're happy with the size of the crystals, take them out and start over with a couple of new eggshells.
The giant one in the bottom right, below, soaked for three or four days.

You can keep reheating and re-saturating this alum solution until you're sick of it. When that happens, pour it down the drain, wash your bowl, and be done. Alum is an edible pickling spice, so it's completely safe.
But don't go eating a bunch of it or anything. Safe and healthy are two different things.
The slideshow below has up-close pictures of most of our crystal geodes. Allie attacked them before I'd gotten to take pictures of all of them, and I forgot to go back and finish.
On that note, they all survived Allie's attack, so the crystals are fairly durable once they've dried.
Alum Crystal Science
I didn't tell Grace most of what's below. I share it now because I assume you're not 5, and you have a longer attention span that she does.
Alum crystals are awesome to grow because many different factors affect their crystal growth.
- You can experiment with cooling rate - let the solution cool normally, cool it faster with a few pieces of ice, cool it really fast with a lot of ice (be careful if you're using a glass bowl - freezing a glass bowl full of hot liquid is a recipe for a broken bowl and giant mess), cool it super slow by keeping the solution in a pan on the stove at a very low temperature. One of the above will result in a few huge crystals and one in scads of tiny crystals.
- All substances have a preferred crystal shape, depending on the alignment of the atoms or molecules that make it up. Potassium aluminum sulfate's crystal shape is isometric. In other words, this alum likes to make octahedrons. (Think two pyramids fused at their bases.) If you look at my slideshow, you will see very few octahedrons.
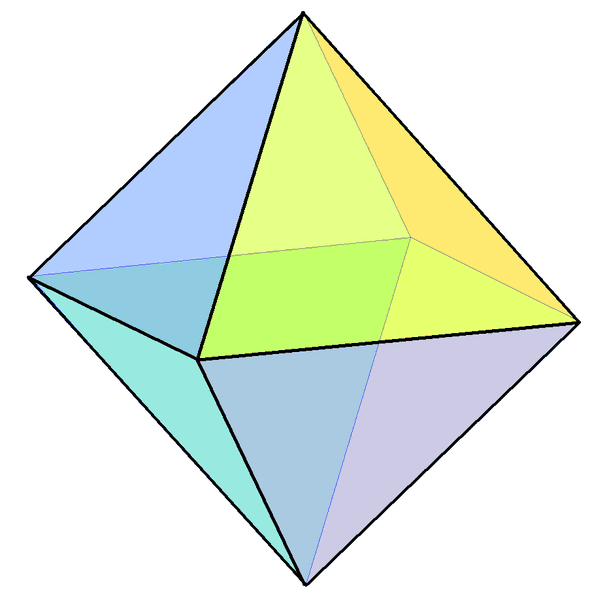
The catch is that crystals can only grow in their preferred crystal shape if they aren't squished. If they're crowded (as yours will most likely be), they grow into whatever space is available. It's not unusual to see almost-trapezoidal crystals like the one below where the octahedrons have interfered with one another. You'll see all sorts of other shapes, too.









Ana Luisa says
They are beautiful
Ana Luisa says
I will tell my sis so she can help me do it ; )
Maria Julia says
Hi I'm Ana Luisas sister
Maria Julia says
🙂
Ana Luisa says
😉
Ana Luisa says
;):)
Katherine says
Did this with my first grader for the school science fair. He loved it, it turned out great, and the crystals were awesome. It was a hit with the kids, parents, and teachers. We cited your website on his poster and I've had a number of parents tell me they have visited it to make geodes at their house. THANK YOU!
Katherine says
FYI, I ordered Jacquard alum through Amazon.
Tara Ziegmont says
Wow, that's awesome! Thank you so much for the recommendation!
Florence says
I love your blog! (chemistry teacher)
Tara Ziegmont says
Thanks 🙂
Takeshi says
when you drop the egg shells in, does the liquid need to be hot? cold? or it doesn't matter.
Tara Ziegmont says
It does matter. The liquid should be hot. The crystals will form as it cools.
Nicole says
As I am reading all the post, I realize that the alum I bought from the spice isle will not be successful for this project! We just did the first step tonight-glue and alum. I do not have enough time to order the alum online. Is the next best thing borax? We have to complete the project for Thursday! Any advice?
Tara Ziegmont says
I've never done borax crystals, but I have had good results with Epsom salts crystals. Overnight is a very tight squeeze though; I don't know if the crystals will have time to form.
gail says
What's the best way to preserve crystals? I've read that clear nail polish isn't a great idea.
Thanks
Tara Ziegmont says
You don't need to do anything to them. They're quite sturdy and will last pretty much forever if you don't knock them around.
Ashley says
What sort of filter did you use to get the extra bits out of the solution?